zinc stearate reaction
This chemical is an example of metal soaps and is insoluble in water and polar solvents such as alcohol. Caprate 050 Laurate 25 Palmitate 28-57 Stearate 28-68 Myristic 3-5 Pentadecanoic up to 050 Margaric Acid up to 2 Oleic acid up to 050 Arachidic and Behenic acid 30-40 Lauric Acid up to 050 Typical.
Catalysts Free Full Text Zinc Based Curing Activators New Trends For Reducing Zinc Content In Rubber Vulcanization Process Html
Solid Solutions of Zinc Stearate and Zinc Oleate.

. It is widely used as a release agent for the production of many kinds of objects. Zinc stearate has different ratios of palmitic and stearic acids. The preparation method of zinc stearate includes the following steps that firstly liquid stearic acid is delivered into a reaction kettle through a delivery pump for stirring heating wherein the stirring time is 20-28 min and the heating temperature is 120-150 DEG C.
It is an activator for accelerated rubber sulfur vulcanization. As discovered in the early days of v. The reaction was NaSt and zinc sulfate.
Zinc stearate is an organic substance with a chemical formula of C36H70O4Zn. Zinc oxide doesnt react with stearic acid. The reaction equation is.
These applications exploit its non-stick properties. The invention discloses a zinc stearate production technology. Zinc Stearate is a material that is easily soluble in water ultra-fine and with good dispersion compatibility.
Emits acrid smoke and fumes of ZnO when heated to decomposition Hazardous Chemicals Desk Reference p. Mixed SaturatedUnsaturated Alkyl-Chain Assemblies. A catalyst paste containing calcium hydroxide zinc oxide and zinc stearate in ethylene toluene sulfonamide reacts with a base paste containing calcium tungstate calcium phosphate and zinc oxide in glycol salicylate to form an amorphous calcium disalicylate.
Considering the molar masses of each chemical substance in the reaction gmol -1 Zn 814 gmol -1 stearic acid 2845 gmol -1 and water 18 gmol -1 and the respective stoichiometric. Water and zinc stearate were formed. Na 909 C 6614 O 1445 also carried out at 10 excess sodium stearate and 10 wt and Zn 3927 S 1539 O 4068 wt values excess zinc sulfate.
Safe handling of zinc stearate is discussed. The aqueous emulsion of zinc. Rubber polyurethane polyester processing system powder metallurgy.
The technology comprises the following steps. Zinc stearate is produced from various reactions including the reaction of zinc oxide and stearic acid which is shown in the following. For zinc and zinc oxide the first reaction zone began at about 160 C and extended to 280290 C.
Mainly used as lubricant and release agent for styrene resin phenolic resin and amino resin. Toluene helped zinc stearate to turn into the corresponding methyl esters by making zinc stearate partly soluble in a raised temperature as well as making the reaction product soluble. Stearic acid reacts with potassium hydroxide to form water and potassium stearate.
The alkaline pH aids in preventing bacterial invasion. From 280 to 375 C zinc stearate was present in the reaction products of the acid with both zinc metal and zinc oxide. Incompatible with oxidizing agents dilute acids.
Belongs to the Following Reactive Group s Esters Sulfate Esters Phosphate Esters Thiophosphate Esters and Borate Esters. Secondly zinc oxide is added into the reaction kettle in three times and a reaction is carried out at the. It is a white powder and is insoluble in water.
Introduction Fatty acid metal salts or soaps play an important role as a process aid. While the melting point of the zinc stearate prepared by. Chmical reaction when stearic acid add KOH.
The Journal of Physical Chemistry B 2007 111 19 5212-5217. Melting of Saturated Fatty Acid Zinc Soaps. ZINC STEARATE is non-flammable but combustible.
In the fusion process an increase in mixing rate decreased the induction time occurring at the beginning of the reaction. The invention belongs to the technical field of chemical engineering and particularly relates to high-quality zinc stearate prepared from glyceryl tristearate. These salts are produced from a reaction with stearic.
For the purpose of this paper we will refer to them as salts. 2 zinc oxide and a catalyst are added stepwise. Since the byproduct Na2SO4 was were obtained.
A preparation technology of zinc stearate comprises the following steps that 1 glyceryl tristearate and water are hydrolyzed under the action of a catalyst and an antioxidant. Raising temperature from room temperature RT to 45C or 90C allowed the reaction to proceed faster. These stearates are viewed as salts or soaps in general terms.
At the same time it also has the function of vulcanization activator and softener in rubber. It is valued by manufacturers because of the delicate feeling it gives to the finished products good heat resistance excellent transparency yellowing resistance quick-drying and increased sandability. At 600 C no acid soap was detected.
Adding zinc oxide four times and reacting at a temperature of 160DEG C under a. Method of producing zinc stearate involves reacting stearic acid and zinc hydroxide with heating and intense stirring followed by heat treatment filtration drying and packaging. Conveying liquid stearic acid by a pump allowing the liquid stearic acid to go through a flow meter for metering and then enter a zinc stearate reaction vessel stirring and heating.
Stearate chemical properties physical properties and the application in rubber. When reacting stearic acid and zinc hydroxide hydrochloric acid is. In cosmetics zinc stearate is a lubricant and thickening agent used to improve texture.

Zinc Stearate Technical Grade 557 05 1
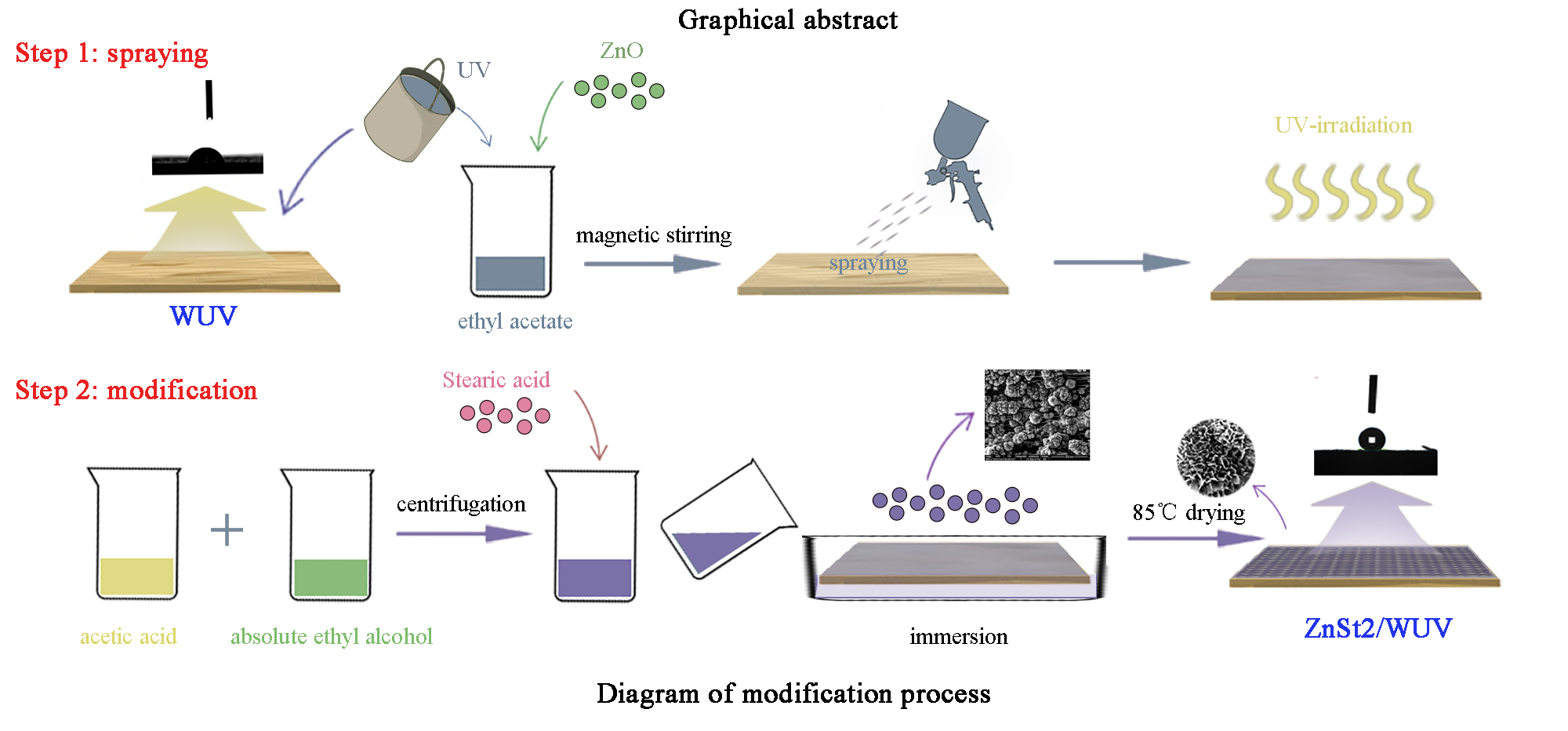
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Special Role For Zinc Stearate And Octadecene In The Synthesis Of Luminescent Znse Nanocrystals Chemistry Of Materials

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Ion Exchange Between Silanol Groups And Zinc Stearate On Silica Surface 28 Download Scientific Diagram

Scielo Brasil Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram
Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Effect Of Synthesized Zinc Stearate On The Properties Of Natural Rubber Vulcanizates In The Absence And Presence Of Some Fillers Sciencedirect

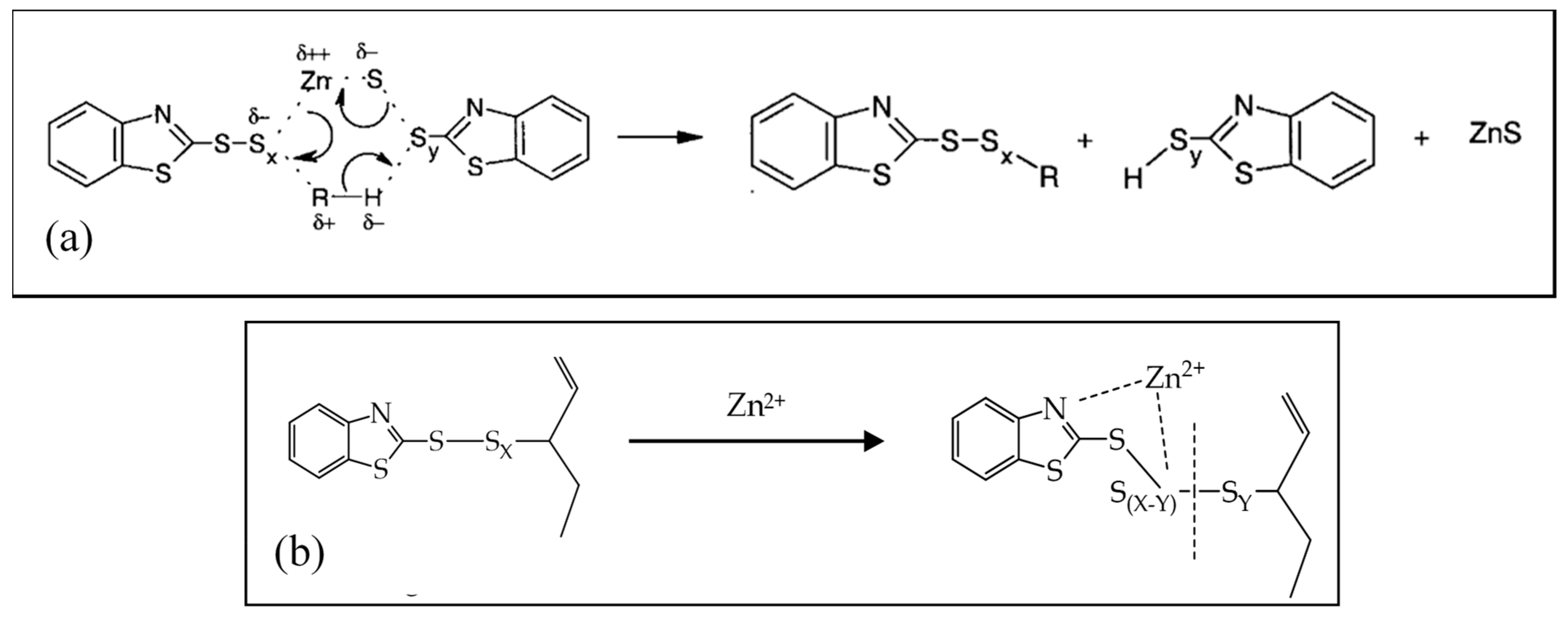
Zinc Stearate Formation And Vulcanization Mechanisms For Step 1 Download Scientific Diagram

Scielo Brasil Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract

Pdf Zinc Stearate Production By Precipitation And Fusion Processes Semantic Scholar
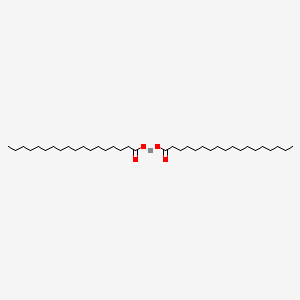
Zinc Stearate C36h70o4zn Pubchem
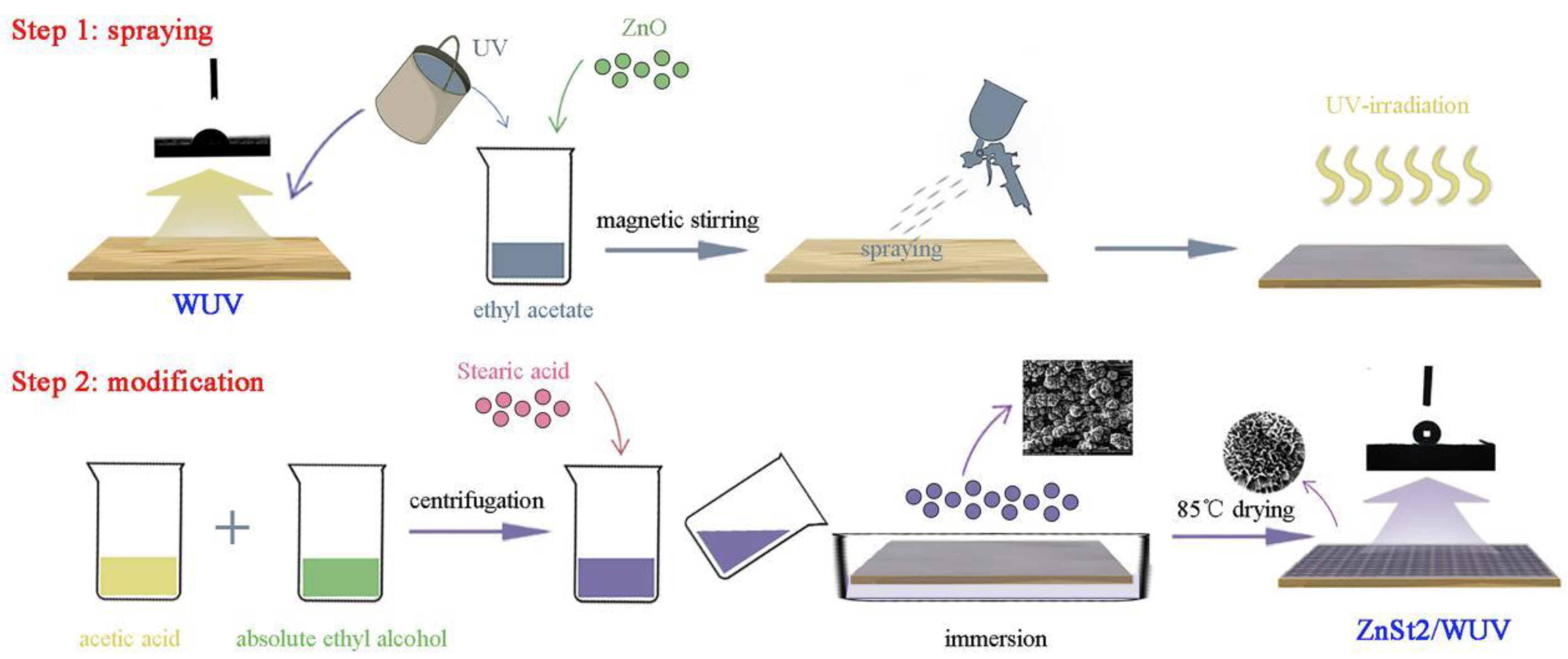
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Influence Of Two Different Alcohols In The Esterification Of Fatty Acids Over Layered Zinc Stearate Palmitate Sciencedirect

Tga Dsc Curves Of Zinc Stearate Reveal A Relative Thermal Equilibrium Download Scientific Diagram

0 Response to "zinc stearate reaction"
Post a Comment